
#Keynote 407 trial
A similar finding was observed when modeling best fitting parametric curves with additional trial follow-up in the present analysis. In earlier cost-effectiveness model publications for the KN189 and KN407 indications 2, 3, we reported that traditional parametric extrapolation of overall survival beyond the trial period resulted in annual mortality risks for the chemotherapy arm that greatly exceeded population-based mortality risks for metastatic NSCLC patients within SEER during the period prior to the availability of pembrolizumab, when chemotherapy was the predominant available treatment. For completeness, for non-squamous metastatic patients, data from the KN021G 9 (data cut-off) phase 2 trial preceding KN189 were also included within the indirect treatment comparison and pooled with that from KN189. Beyond the trial duration, survival data is extrapolated and, in the model base case, a 20-year time horizon is used for practical considerations, as <5% of non-squamous and squamous patients initiating pembrolizumab + chemotherapy are modeled to survive beyond this time point.įor comparisons to pembrolizumab monotherapy, as described in greater detail in earlier work 2, 3, an individual patient data (IPD)-based indirect treatment comparison was undertaken using final analysis data from the KN024 (Jdata cut-off as previously used) and KN042 (Septemdata cut-off updated from Februdata cut-off previously used) trials. The median follow-up available in KN189 and KN407 from the start of follow-up for each patient to the and cut-off dates was 31.0 months (range = 26.5–38.8) and 23.3 months (range = 16.4–32.7), respectively. Paclitaxel 200 mg/m 2 every 3 weeks or nab-Paclitaxel 100 mg/m 2 once weekly, over four 3-week cycles.
Pembrolizumab 200 mg once every 3 weeks, for up to 35 cycles (2 years).Ĭarboplatin area under the curve (AUC) 6, every 3 weeks for four cycles.

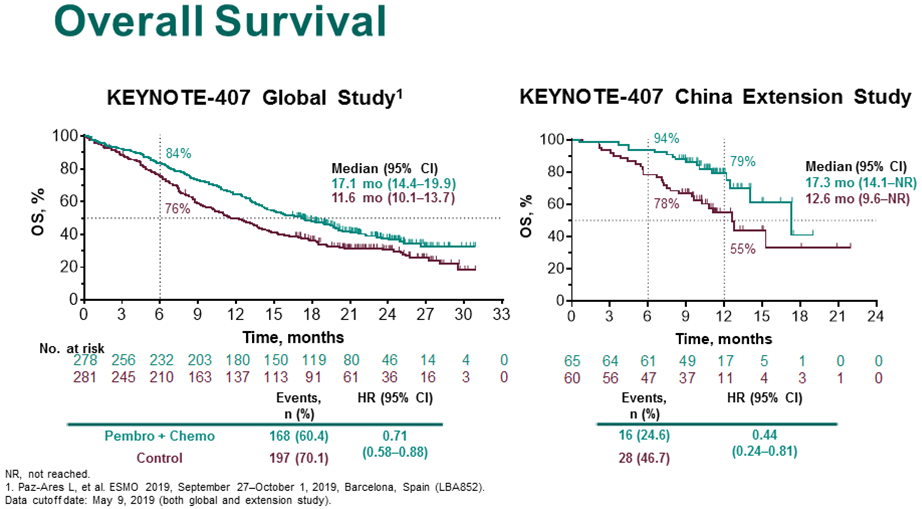
Pemetrexed 500 mg/m 2 every 3 weeks for four cycles, followed by maintenance Pemetrexed. Specific treatment regimens modeled are:Ĭarboplatin area under the curve (AUC) 5 or Cisplatin 75 mg/m 2, every 3 weeks for four cycles. Indirect comparison (ITC) to pembrolizumab monotherapy is evaluated within subgroup analyses of patients with PD-L1 ≥ 50% and 1–49%, as these encompass the currently approved first-line US indication for monotherapy, based on the KN024 6 (PD-L1 ≥ 50%) and KN042 7, 8 (PD-L1 ≥ 1%) trials. The primary comparators in the models are the chemotherapy arms in KN189/KN407. The present evaluation updates previous cost-effectiveness analysis findings based on protocol-specified final analysis data from these trials. At 2-year follow-up for KN189, for non-squamous patients, overall survival was 45.7% in the pembrolizumab + chemotherapy arm, as compared to 27.3% in the chemotherapy arm, while in KN407, for squamous patients, overall survival was 37.5% and 30.6%, respectively, at this time point.
At the final analyses, a substantial improvement in overall survival with pembrolizumab + chemotherapy vs chemotherapy was observed in both trials, with hazard ratios (HR) of 0.56 (95% confidence interval = 0.46–0.69) and 0.71 (0.58–0.88) for patients with non-squamous and squamous histologies in KN189 and KN407, respectively. More recently, updated protocol-specified final clinical trial analyses have been reported, reflecting an additional 1.1 years (KN407) and 1.5 years (KN189) of patient follow-up 4, 5. Previously we reported on the cost-effectiveness of pembrolizumab + chemotherapy versus chemotherapy Phase 3 trial comparators, and pembrolizumab monotherapy, in metastatic non-squamous 2 and squamous 3 US NSCLC patients based on interim trial analysis data (non-squamous KEYNOTE-189 and squamous KEYNOTE-407 trials), with median follow-up time from patient enrollment to the data cut-off date of ∼1 year. In metastatic NSCLC, pembrolizumab monotherapy has also been approved in the US for treatment of patients whose tumors have programmed death-ligand 1 (PD-L1) expression (Tumor Proportion Score ≥ 1%) without EGFR/ALK aberrations 1. Specifically, pembrolizumab in combination with carboplatin/cisplatin (platinum) and pemetrexed has been approved for US use in metastatic non-squamous patients with no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genomic tumor aberrations, and pembrolizumab + carboplatin and nab-paclitaxel/paclitaxel has been approved in metastatic squamous NSCLC 1. Pembrolizumab (Keytruda) in combination with chemotherapy is approved in the US and many other countries for the treatment of metastatic non-small cell lung cancer (NSCLC).


 0 kommentar(er)
0 kommentar(er)
